Articles from NanoVibronix, Inc.

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in non-invasive therapeutic systems, today announced that it will change its corporate name from NanoVibronix, Inc. to ENvue Medical, Inc., effective December 12, 2025, to better reflect its new strategic direction and primary focus on its ENvue® feeding-tube placement system (“ENvue”). The Company’s ticker for its common stock, listed on the Nasdaq Capital Market, will also change to “FEED,” which will begin trading under the new symbol beginning with the market open on the same day, December 12, 2025.
By NanoVibronix, Inc. · Via Business Wire · December 12, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) ("NanoVibronix" or the "Company"), a medical technology company specializing in non-invasive therapeutic systems, today issued a letter to shareholders, highlighting the Company’s strategic vision, market opportunities and a rebranding initiative.
By NanoVibronix, Inc. · Via Business Wire · November 19, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in non-invasive therapeutic systems, today announced the launch of Oscar, an advanced training aid, introduced through its ENvue Medical (“ENvue” or “ENvue Medical”) division. Oscar is designed to provide clinicians and educators with a standardized, repeatable approach to practicing naso-enteral feeding procedures, addressing a key barrier to adoption of new and current technologies.
By NanoVibronix, Inc. · Via Business Wire · September 18, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in non-invasive therapeutic systems, today announced that it has entered into a definitive agreement with a single institutional investor for the purchase and sale of 291,204 shares of its common stock (or common stock equivalents), at an offering price of $7.01 per share of common stock (or per common stock equivalent) in a registered direct offering priced at-the-market under Nasdaq rules. The closing of the offering is expected to occur on or about September 17, 2025, subject to the satisfaction of customary closing conditions.
By NanoVibronix, Inc. · Via Business Wire · September 16, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in non-invasive therapeutic systems, today announced that the United States Patent and Trademark Office issued U.S. Patent No. 12,409,105 B2, titled “Insertion Device Positioning Guidance System and Method” on September 9, 2025 to ENvue Medical Holdings LLC (formerly ENvue Medical Holdings, Corp.), a wholly-owned subsidiary of the Company (“ENvue” or “ENvue Medical”).
By NanoVibronix, Inc. · Via Business Wire · September 15, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in non-invasive therapeutic systems, today announced that the United States Patent and Trademark Office issued U.S. Patent No. 12,402,953 B2, titled “Insertion Device Positioning Guidance System and Method” on September 2, 2025 to ENvue Medical Holdings LLC (formerly ENvue Medical Holdings, Corp.), a wholly-owned subsidiary of the Company (“ENvue” or “ENvue Medical”). The patent protects the Company’s proprietary method of overlaying electromagnetic (EM) navigation data directly onto real-time medical imaging modalities such as X-ray, CT, ultrasound and MRI.
By NanoVibronix, Inc. · Via Business Wire · September 4, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in non-invasive therapeutic devices, today announced that it intends to effect a reverse stock split of its common stock, par value $0.001 per share (the “common stock”) at a ratio of 1 post-split share for every 10 pre-split shares. The reverse stock split will become effective at 4:05 p.m. ET on Monday, August 11, 2025. The Company’s common stock will continue to be traded on the Nasdaq Capital Market under the symbol NAOV and will begin trading on a split-adjusted basis when the market opens on Tuesday, August 12, 2025. The new CUSIP number for the common stock following the reverse stock split is 63008J884.
By NanoVibronix, Inc. · Via Business Wire · August 8, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in therapeutic devices for enteral feeding, pain management and urological conditions, today announced it has entered into a definitive agreement with an institutional investor for the purchase and sale of 8,889 shares of Series H Convertible Preferred Stock (the “Preferred Stock”) with a total stated value of $8,888,889. The Preferred Stock is convertible into shares of the Company’s common stock, par value $0.001 per share (the “Common Stock”), at an initial conversion price of $1.01, which such conversion price is equal to the closing price as reported on Nasdaq on the trading day immediately prior to signing of the definitive agreements and is subject to customary anti-dilution adjustments. The initial closing is expected to occur on or about July 21, 2025, subject to the satisfaction of customary closing conditions (the “Initial Closing”).
By NanoVibronix, Inc. · Via Business Wire · July 18, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in non-invasive therapeutic systems, today announced the official launch of a next-generation robotic development initiative within its ENvue Medical (“ENvue” or “ENvue Medical”) division. The new platform, ENvue Drive™, is being developed to automate electromagnetic navigation for enteral and vascular access procedures at the bedside.
By NanoVibronix, Inc. · Via Business Wire · June 26, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in non-invasive therapeutic devices, announced today that a regional acute care hospital in upstate New York has signed an agreement to acquire two ENvue™ Navigation Systems through its ENvue Medical division (“ENvue” or “ENvue Medical”).
By NanoVibronix, Inc. · Via Business Wire · June 24, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in therapeutic devices, announced today, through its ENvue Medical Holdings LLC division (“ENvue Medical” or “ENvue”), a new U.S. patent for its pediatric feeding tube guidance system. U.S. Patent No. 12,324,632, titled “Insertion Device Positioning Guidance System,” further strengthens ENvue Medical’s intellectual property portfolio and reflects the Company’s ongoing commitment to innovation in feeding tube placement technologies.
By NanoVibronix, Inc. · Via Business Wire · June 11, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in therapeutic devices, today announced the retirement of Brian Murphy as Chief Executive Officer, effective immediately. The Company’s Board of Directors has appointed Doron Besser, CEO of ENvue Medical Holdings, LLC (“ENvue”), a wholly-owned subsidiary of the Company, as NanoVibronix’s new CEO, also effective immediately. Mr. Murphy will remain on the Company’s Board of Directors as a director. His continued service will support governance continuity at the Board level.
By NanoVibronix, Inc. · Via Business Wire · June 4, 2025

NanoVibronix, Inc. (the “Company”) (NASDAQ: NAOV) confirmed that a series of posts made earlier today with respect to a $26 million registered direct offering of common stock of the Company priced at $0.45 under Nasdaq rules was falsely issued by Flash Alert and FintelAlerts. The Company has not priced and is not consummating any registered direct offering. The Company is actively investigating this misinformation and will take necessary actions to protect its stockholders and its reputation.
By NanoVibronix, Inc. · Via Business Wire · May 28, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in therapeutic devices, announced today, through its ENvue Medical Holdings LLC division (“ENvue Medical”), that a major 420-bed acute care hospital located in the greater New Orleans area has selected the ENvue™ Navigation Platform (the “ENvue System”) to support adult feeding tube procedures. The hospital will transition from traditional blind insertions to real-time, electromagnetic-guided placement using the ENvue System.
By NanoVibronix, Inc. · Via Business Wire · May 28, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in therapeutic devices, today announced the closing of its previously announced underwritten public offering of 400,000 shares of the Company’s Series G Convertible Preferred Stock (“Preferred Stock”), with a par value $0.001 per share and a stated value equal to $25, and Warrants to purchase up to 4,901,982 shares of common stock, par value $0.001 per share (the “Common Stock”) of the Company at an exercise price of $2.04 per share (the “Warrants”). The combined public offering price of each share of Preferred Stock together with an accompanying Warrant is $25.00. The Warrants have a term of five years from the initial issuance date and are exercisable immediately upon issuance. The holders of the Preferred Stock are entitled to receive cumulative dividends at the rate per share of 9% per annum of the stated value per share until the fifth anniversary of the date of issuance of the Preferred Stock, which such dividends may be paid, at the Company’s option, in up to an aggregate of 2,205,883 shares of Common Stock.
By NanoVibronix, Inc. · Via Business Wire · May 16, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in therapeutic devices, today announced the pricing of an underwritten public offering of approximately 400,000 shares of the Company’s Series G Convertible Preferred Stock (“Preferred Stock”), par value $0.001 per share and Warrants to purchase up to 4,901,961 shares of common stock, par value $0.001 per share (the “Common Stock”) of the Company at an exercise price of $2.04 per share (the “Warrants”). The combined public offering price of each share of Preferred Stock together with an accompanying Warrant is $25.00. The closing of the public offering is expected to occur on or about May 19, 2025, subject to the satisfaction of customary closing conditions.
By NanoVibronix, Inc. · Via Business Wire · May 15, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company”), a medical technology company specializing in therapeutic devices, today announced findings from a recent retrospective case series evaluating the clinical impact and patient experience of the UroShield™ system.
By NanoVibronix, Inc. · Via Business Wire · May 12, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company” or “NanoVibronix”), a medical technology company specializing in non-invasive therapeutic devices, today announced that on April 9, 2025, Nasdaq formally notified the Company that it has evidenced full compliance with all criteria for continued listing on The Nasdaq Capital Market, including the $1.00 bid price and $2.5 million stockholders’ equity requirements. Accordingly, the previously disclosed Nasdaq listing matter has been closed. Pursuant to Listing Rule 5815(d)(4)(B), the Company will be subject to a Mandatory Panel Monitor until April 9, 2026.
By NanoVibronix, Inc. · Via Business Wire · April 11, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company” or “NanoVibronix”), a medical technology company specializing in non-invasive therapeutic devices, today announced the renewal and expansion of its exclusive three-year distribution agreement with Dukehill Healthcare Pty Ltd. (“Dukehill”).
By NanoVibronix, Inc. · Via Business Wire · April 1, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company”), a medical technology company specializing in non-invasive therapeutic devices, today announced that an independent health service study of its UroShield® is published in The Australian and New Zealand Continence Journal.
By NanoVibronix, Inc. · Via Business Wire · March 14, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (“NanoVibronix” or the “Company”), a medical technology company specializing in non-invasive therapeutic devices, today announced that it intends to effect a reverse stock split of its common stock, par value $0.001 per share (the “common stock”) at a ratio of 1 post-split share for every 11 pre-split shares. The reverse stock split will become effective at 4:05 p.m. on Thursday, March 13, 2025. The Company’s common stock will continue to be traded on the Nasdaq Capital Market under the symbol NAOV and will begin trading on a split-adjusted basis when the market opens on Friday, March 14, 2025. The new CUSIP number for the common stock following the reverse stock split is 63008J702.
By NanoVibronix, Inc. · Via Business Wire · March 12, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company”), a medical technology company specializing in non-invasive therapeutic devices, today announced the completion of the acquisition of ENvue Medical Holdings Corp. (“ENvue”) (the “Acquisition”), a privately-held, innovative leader in enteral feeding solutions. This strategic transaction will combine the strengths of both companies, creating a platform for growth and expanded market reach in the medical device sector. The Company believes that the Acquisition will strengthen the combined company’s market position in enteral feeding technology and therapeutic medical devices, as ENvue’s proprietary technology aligns with the Company’s commitment to patient safety and advanced medical solutions and the combined company is expected to benefit from a broader commercial platform, enhanced distribution and operational efficiencies.
By NanoVibronix, Inc. · Via Business Wire · February 14, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company”), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced a third-party contractor engaged by the Company has completed the design phase for the development of the Company’s next generation PainShield® and UroShield® devices.
By NanoVibronix, Inc. · Via Business Wire · January 7, 2025

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company”), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced the successful completion of the first phase of a Randomized Control Trial study of UroShield® by researchers at the University of Michigan (“UM”).
By NanoVibronix, Inc. · Via Business Wire · January 6, 2025

NanoVibronix, Inc., (NASDAQ: NAOV) (the “Company”), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced it has renewed its exclusive distribution agreement with Ultra Pain Products, Inc. (“UPPI”) for the distribution of the Company’s PainShield for another five years.
By NanoVibronix, Inc. · Via Business Wire · December 11, 2024

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company”), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today provided an update on recent business developments.
By NanoVibronix, Inc. · Via Business Wire · November 12, 2024

NanoVibronix, Inc., (NASDAQ: NAOV) (the “Company”), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (“SAW”) Portable Ultrasonic Therapeutic Devices, today announced it has signed a non-binding term sheet for a license and supply agreement with APOGEPHA Arzneimittel GmbH (“APOGEPHA”), a German pharmaceutical company, which specializes in the development, marketing, sales and distribution of urology products and services. Pursuant to the term sheet, the Company and APOHEPHA intend to enter into a definitive agreement, pursuant to which, APOHEPHA will distribute UroShield throughout Europe.
By NanoVibronix, Inc. · Via Business Wire · October 9, 2024

NanoVibronix, Inc., (NASDAQ: NAOV) (the “Company”), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced it has signed a letter of intent with Medici Medical LTD (“Medici”), a leading distributor of medical products in the Israeli healthcare market, to explore the opportunities for distribution of the Company’s UroShield device in Israel.
By NanoVibronix, Inc. · Via Business Wire · September 23, 2024

NanoVibronix, Inc. (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today issued a letter to its stockholders from its Chief Executive Officer, Brian Murphy, providing a review of the second quarter financials and recent business developments.
By NanoVibronix, Inc. · Via Business Wire · August 21, 2024

NanoVibronix, Inc. (Nasdaq: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that the University of Michigan will begin the pilot phase of its clinical trial of UroShield this week. The “pilot” phase of the study is the first component of the broader study.
By NanoVibronix, Inc. · Via Business Wire · May 22, 2024

NanoVibronix, Inc. (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today issued a letter to its stockholders from its Chief Executive Officer, Brian Murphy, providing a review of the first quarter of 2024 and recent business developments.
By NanoVibronix, Inc. · Via Business Wire · May 16, 2024

NanoVibronix, Inc. (Nasdaq: NAOV) (“NanoVibronix,” or the “Company”), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that it has entered into a distribution agreement with CB Medical, LLC (“CB Medical”) for the sale and distribution of the Company’s UroShield.
By NanoVibronix, Inc. · Via Business Wire · May 14, 2024

NanoVibronix, Inc. (Nasdaq: NAOV) (“NanoVibronix,” or the “Company”), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that effective May 1, 2024, the Company’s PainShield product will be included in the U.S. Department of Veteran Affairs’ (“VA”) Federal Supply Schedule (“FSS”), a program that supports the healthcare acquisition needs of the VA and other government agencies.
By NanoVibronix, Inc. · Via Business Wire · May 1, 2024

NanoVibronix, Inc., (NASDAQ: NAOV)(“NanoVibronix”), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today issued a letter to stockholders from its Chief Executive Officer, Brian Murphy, providing a review of the fourth quarter and full year 2023 and recent business developments.
By NanoVibronix, Inc. · Via Business Wire · April 9, 2024

NanoVibronix, Inc. (Nasdaq: NAOV) (“NanoVibronix” or the “Company”), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced it has entered into an agreement with Veranex, Inc. (“Veranex”) to assist with the development of the company’s next generation UroShield and PainShield products.
By NanoVibronix, Inc. · Via Business Wire · March 28, 2024

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that its UroShield is featured in Issue 103 of Your Bladder Health Magazine in an article titled ‘UroShield Prevention is better than cure.’ The article highlights the effectiveness of using UroShield in the prevention of catheter associated urinary tract infections (CAUTIs). The article also includes testimonials that detail the positive impact that UroShield has had on the lives of patients.
By NanoVibronix, Inc. · Via Business Wire · December 6, 2023

NanoVibronix, Inc. (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that it has entered into a non-binding letter of intent (the “LOI”) with APOGEPHA Arzneimittel GmbH (“APOGEPHA”) in which both parties will analyze the potential for APOGEPHA to distribute NanoVibronix’s premiere UroShield product in Germany and other European markets.
By NanoVibronix, Inc. · Via Business Wire · December 4, 2023

NanoVibronix, Inc. (Nasdaq: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced it has signed a Research Agreement with the Regents of the University of Michigan for a Randomized Control Trial (“RCT”) study of UroShield.
By NanoVibronix, Inc. · Via Business Wire · November 28, 2023

NanoVibronix, Inc., (NASDAQ: NAOV) (the “Company”), a medical device company that produces the UroShield® and PainShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that on November 20, 2023, the Company received official notice from The Nasdaq Stock Market LLC that the Company has evidenced compliance with all applicable criteria for continued listing on The Nasdaq Capital Market, including the minimum stockholders’ equity requirement of $2.5 million. Accordingly, the previously disclosed listing matter has been closed.
By NanoVibronix, Inc. · Via Business Wire · November 21, 2023

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today issued a letter to shareholders from its Chief Executive Officer, Brian Murphy, providing a review of the third quarter 2023 and recent business developments.
By NanoVibronix, Inc. · Via Business Wire · November 14, 2023

NanoVibronix, Inc. (Nasdaq: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that effective November 1, its UroShield actuators are eligible for reimbursement on NHS Prescription Services’ Drug Tariff.
By NanoVibronix, Inc. · Via Business Wire · November 7, 2023

NanoVibronix, Inc. (Nasdaq: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that its application for UroShield actuators to be available on NHS Prescription Services’ Drug Tariff has been approved.
By NanoVibronix, Inc. · Via Business Wire · September 27, 2023

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that Dr. Sandra Wilks is scheduled to present the findings from her study of UroShield® at the International Continence Society (ICS) 2023 Conference in Toronto on Wednesday, September 27.
By NanoVibronix, Inc. · Via Business Wire · September 6, 2023

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that it has entered into definitive agreements for the purchase and sale of an aggregate of 2,906,977 shares of its common stock (or common stock equivalents in lieu thereof), series A-1 warrants to purchase up to 2,906,977 shares of common stock and series A-2 warrants to purchase up to 2,906,977 shares of common stock, at a purchase price of $1.72 per share of common stock (or common stock equivalent in lieu thereof) and accompanying warrants, in a private placement priced at-the-market under Nasdaq rules.
By NanoVibronix, Inc. · Via Business Wire · August 30, 2023

NanoVibronix, Inc. (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today cited positive reported outcomes from a study of UroShield® that was conducted at the University of Southampton in the United Kingdom.
By NanoVibronix, Inc. · Via Business Wire · August 30, 2023

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today provided a response to the recently published payment determination by the Centers for Medicare & Medicaid Services (“CMS”) regarding the company’s PainShield device.
By NanoVibronix, Inc. · Via Business Wire · August 29, 2023

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today provided an update on its efforts to expand distribution of its UroShield family of products.
By NanoVibronix, Inc. · Via Business Wire · August 28, 2023

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that it has conditionally extended its distribution agreement with Ultra Pain Products, Inc. (“UPPI”) for the company’s PainShield® and PainShield Plus®.
By NanoVibronix, Inc. · Via Business Wire · August 23, 2023

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that it has entered into a distribution agreement with Mio-Guard, LLC (“Mio-Guard”) for the sale and distribution of its PainShield MD product.
By NanoVibronix, Inc. · Via Business Wire · June 14, 2023

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today issued a letter to shareholders from its Chief Executive Officer, Brian Murphy, providing a review of the first quarter 2023 and recent business developments.
By NanoVibronix, Inc. · Via Business Wire · May 18, 2023

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company that produces the UroShield®, PainShield® and WoundShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today issued a letter to shareholders from its Chief Executive Officer, Brian Murphy, providing a year in review and vision for 2023.
By NanoVibronix, Inc. · Via Business Wire · April 18, 2023

NanoVibronix, Inc., (NASDAQ: NAOV) (the “Company”), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (“SAW”) technology, today announced that independent life expectancy testing performed by Carmel Labs in Israel confirm that its PainShield met all tested functionalities.
By NanoVibronix, Inc. · Via Business Wire · March 28, 2023

NanoVibronix, Inc., (NASDAQ: NAOV) (the “Company”), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (“SAW”) technology, today announced it has filed a new provisional patent application with the United States Patent and Trademark Office (“USPTO”) entitled “Multiple Frequency Surface Acoustic Waves for Internal Medical Device” (the "Patent Application") related to its UroShield.
By NanoVibronix, Inc. · Via Business Wire · March 21, 2023

NanoVibronix, Inc., (NASDAQ: NAOV) (the “Company”), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced the positive evaluation results for its UroShield device, presented at a recent medical conference by clinicians from the Royal National Orthopaedic Hospital (“RNOH”).
By NanoVibronix, Inc. · Via Business Wire · March 14, 2023

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company”), a medical device company that produces the UroShield® and PainShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced that on February 28, 2023, the Company received official notice from The Nasdaq Stock Market LLC that the Company has evidenced compliance with all applicable criteria for continued listing on The Nasdaq Capital Market, including the $1.00 bid price requirement. Accordingly, the previously announced Nasdaq listing matter has been closed.
By NanoVibronix, Inc. · Via Business Wire · March 3, 2023

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced that it intends to launch a month to month rental program on March 1, 2023 for its lead products, PainShield and UroShield.
By NanoVibronix, Inc. · Via Business Wire · February 22, 2023

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company”), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced that it intends to effect a reverse stock split of its common stock at a ratio of 1 post-split share for every 20 pre-split shares. The reverse stock split will become effective at 4:05 p.m. on Wednesday, February 8, 2023. The Company’s common stock will continue to be traded on the Nasdaq Capital Market under the symbol NAOV and will begin trading on a split-adjusted basis when the market opens on Thursday, February 9, 2023. The new CUSIP number for the common stock following the reverse stock split is 63008J603.
By NanoVibronix, Inc. · Via Business Wire · February 8, 2023
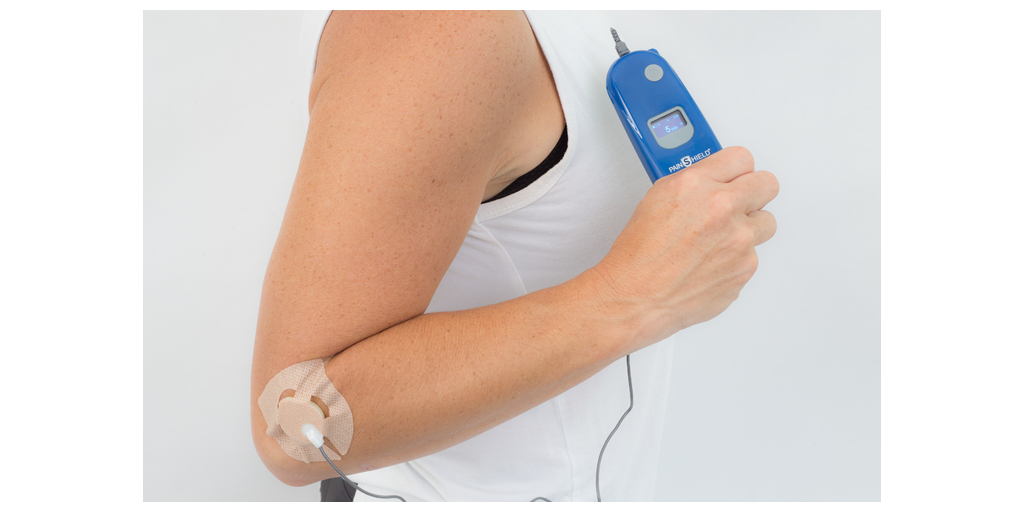
NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company”), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today provided an update on the efforts to expand access to the Company’s PainShield product.
By NanoVibronix, Inc. · Via Business Wire · January 18, 2023

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company”), a medical device company utilizing the Company’s proprietary and patented low intensity surface acoustic wave (SAW) technology, announced that the Company’s 2022 annual meeting of stockholders (the “Annual Meeting”) was held today virtually and broadcast live at www.virtualshareholdermeeting.com/NAOV2022.
By NanoVibronix, Inc. · Via Business Wire · December 15, 2022

NanoVibronix, Inc. (NASDAQ: NAOV), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced that it has partnered with Peak Medical, Ltd., a continence care patient focused company based in the United Kingdom.
By NanoVibronix, Inc. · Via Business Wire · December 13, 2022

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company”), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced the closing of its previously announced registered direct offering priced at-the-market under Nasdaq rules, of 4,800,000 shares of its common stock at a purchase price of $0.50 per share.
By NanoVibronix, Inc. · Via Business Wire · December 1, 2022

NanoVibronix, Inc. (NASDAQ: NAOV) (the “Company”), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced that it has entered into a securities purchase agreement with several institutional investors for the purchase and sale, in a registered direct offering priced at-the-market under Nasdaq rules, of 4,800,000 shares of its common stock (or pre-funded warrants in lieu of thereof) at a purchase price of $0.50 per share (or pre-funded warrant in lieu thereof). The offering is expected to close on or about December 1, 2022, subject to satisfaction of customary closing conditions.
By NanoVibronix, Inc. · Via Business Wire · November 29, 2022

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its PainShield® MD PLUS, its dual-actuator ultrasound pain therapy device.
By NanoVibronix, Inc. · Via Business Wire · November 28, 2022

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced interim results of its U.K. clinical study for UroShield that is being conducted by The University of Southampton and the National Biofilms Innovation Centre.
By NanoVibronix, Inc. · Via Business Wire · November 16, 2022

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today provided the following business update in a letter to shareholders from its Chief Executive Officer, Brian Murphy:
By NanoVibronix, Inc. · Via Business Wire · November 15, 2022

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced that on October 17, 2022, a Nasdaq Hearings Panel (the “Panel”) granted the Company’s request for continued listing on The Nasdaq Capital Market (“Nasdaq”), subject to the Company’s satisfaction of certain conditions, including the provision of an update to the Panel on December 15, 2022, regarding the Company’s efforts to evidence compliance with the $1.00 bid price requirement for continued listing on Nasdaq. Following the submission of such update, the Panel will consider the Company’s request for a further extension. The Panel has the authority to grant the Company an extension through February 23, 2023, in accordance with the Nasdaq Listing Rules.
By NanoVibronix, Inc. · Via Business Wire · October 19, 2022

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced its UroShield has been approved for sale by the U.K.’s National Health System’s (NHS) internal supply organization, NHS Supply Chain, through a new contract.
By NanoVibronix, Inc. · Via Business Wire · September 23, 2022

NanoVibronix, Inc., (NASDAQ: NAOV) (the “Company”), announced today that its annual meeting of shareholders (the “Annual Meeting”) will take place at 10 a.m. Eastern time on December 15, 2022. The Company will hold the Annual Meeting virtually via the internet.
By NanoVibronix, Inc. · Via Business Wire · September 14, 2022

NanoVibronix, Inc. (NASDAQ: NAOV) (“Nano” or the “Company”), a medical device company utilizing the Company’s proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced that its Board of Directors declared a dividend of one one-thousandth of a share of newly designated Series F Preferred Stock, par value $0.001 per share, for each outstanding share of the Company’s common stock held of record as of 5:00 p.m. Eastern Time on October 14, 2022. The shares of Series F Preferred Stock will be distributed to such recipients at 5:00 p.m. Eastern Time on October 17, 2022. The outstanding shares of Series F Preferred Stock will vote together with the outstanding shares of the Company’s common stock, as a single class, exclusively with respect to a reverse stock split, as well as any proposal to adjourn any meeting of stockholders called for the purpose of voting on the reverse stock split, and will not be entitled to vote on any other matter, except to the extent required under the Delaware General Corporation Law. Subject to certain limitations, each outstanding share of Series F Preferred Stock will have 1,000,000 votes per share (or 1,000 votes per one one-thousandth of a share of Series F Preferred Stock).
By NanoVibronix, Inc. · Via Business Wire · September 14, 2022

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced that, on August 30, 2022, the Company received notice from the Nasdaq Listing Qualifications Staff (the “Staff”) indicating that, based upon the Company’s continued non-compliance with Nasdaq’s minimum bid price requirement as of August 29, 2022, the Company securities would be subject to delisting unless the Company timely requests a hearing before the Nasdaq Hearings Panel (the “Panel”). The Company plans to timely request a hearing, which request will stay any further action by the Staff at least pending the issuance of the Panel’s decision following the hearing and the expiration of any extension that may be granted by the Panel.
By NanoVibronix, Inc. · Via Business Wire · September 2, 2022

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today provided the following business update in a letter to shareholders from its Chief Executive Officer, Brian Murphy:
By NanoVibronix, Inc. · Via Business Wire · August 18, 2022

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced its Australian distributor, DukeHill HC, is launching a series of educational webinars to present the Company’s UroShield technology to clinicians. The series, which will feature clinicians and patients’ experiences, seeks to inform clinicians about the use, benefits and outcomes achieved when using the Company’s device.
By NanoVibronix, Inc. · Via Business Wire · July 12, 2022

NanoVibronix, Inc. (NASDAQ: NAOV), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today announced it is shifting the majority of its manufacturing operations to Singapore in order to allow scalability increase efficiency and address potential supply chain delays.
By NanoVibronix, Inc. · Via Business Wire · June 29, 2022

NanoVibronix, Inc., (NASDAQ: NAOV), a medical device company utilizing the Company's proprietary and patented low intensity surface acoustic wave (SAW) technology, today reported its financial results for the quarter ended March 31, 2022.
By NanoVibronix, Inc. · Via Business Wire · May 17, 2022

NanoVibronix, Inc. (NASDAQ: NAOV), a medical device company that produces the UroShield® and PainShield® Surface Acoustic Wave (SAW) Portable Ultrasonic Therapeutic Devices, today announced it has submitted a 510(k) application to the U.S. Food and Drug Administration (FDA) for its PainShield® MD PLUS, its dual-actuator ultrasound pain therapy device.
By NanoVibronix, Inc. · Via Business Wire · May 4, 2022
